
SOLVED AsH3 vs ClF3. what are the Lewis structure and resonance? Why
A molecule (or polyatomic ion) is polar when one side of the molecule is more positive (or more negative) than the other. This occurs when the polarities of the bonds do not cancel out. For example in CO 2, each carbon-oxygen bond is polar, but CO 2 is a nonpolar molecule .
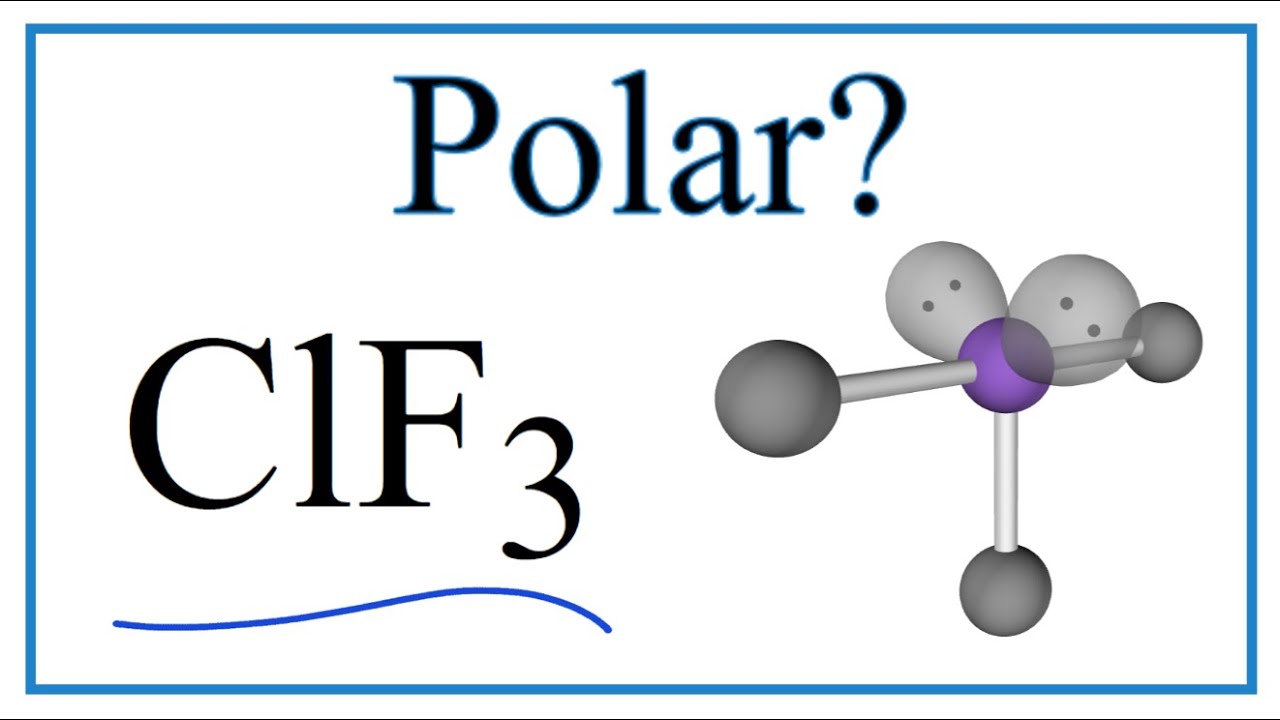
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube
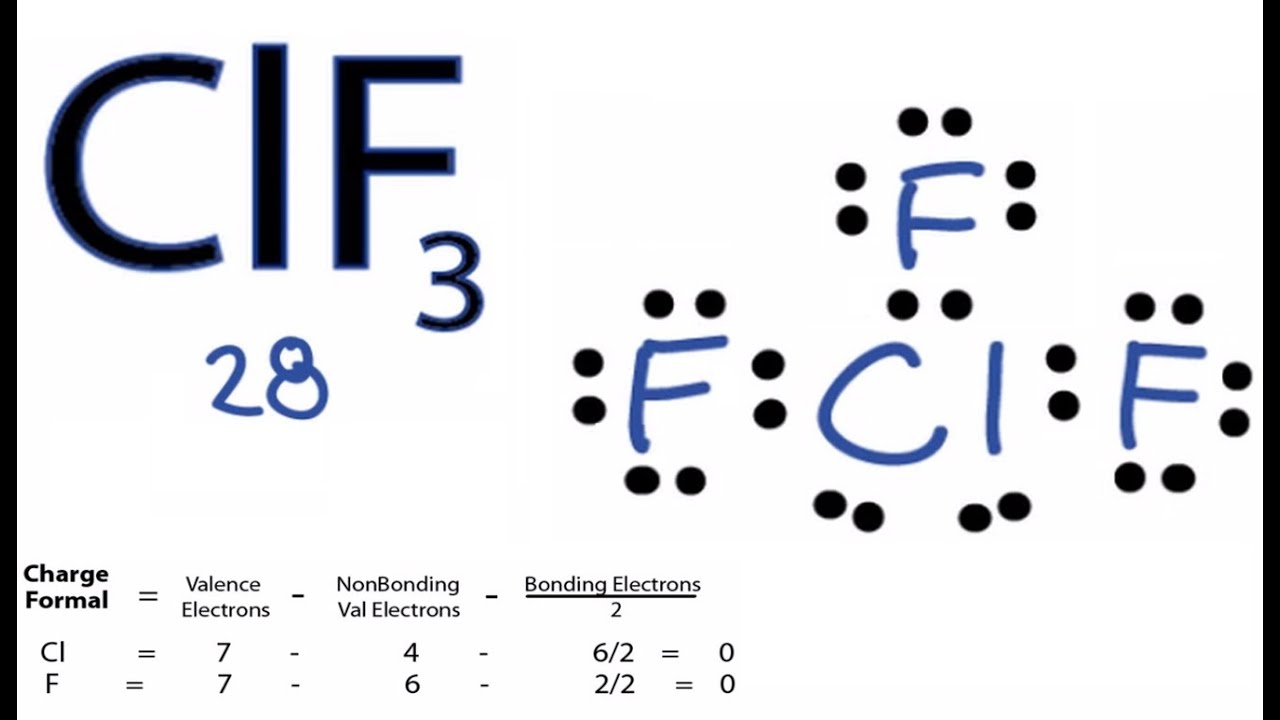
Since there are three fluorine atoms in ClF3, calculate the total valence electrons as follows: 7 (chlorine) + 3 * 7 (fluorine) = 28 valence electrons. 2. Select the Central Atom In ClF3, the central atom is typically the least electronegative atom, which, in this case, is chlorine (Cl). 3. Connect Atoms by Placing Electron Pairs Between Them
Chf3 Polar Or Non Polar
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Free download hd /best overview is ch4 polar or nonpolar science
Chlorine trifluoride is an interhalogen compound with the formula ClF 3.This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). Despite being famous for its extreme oxidation properties and igniting many things, chlorine trifluoride is not combustible itself.
MakeTheBrainHappy Is ClF3 Polar or Nonpolar?
ClF3 or Chlorine Trifluoride is a strong fluorinating agent. To find out whether this molecule is polar or nonpolar, watch this video, where we share our quick and detailed method to.

Discover 66+ draw the structure of clf3 super hot xkldase.edu.vn
Chlorine trifluoride or ClF3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. An interhalogen compound having both Cl and F, it has a density of around 3.79 g/l and a molar mass of 92.45 g/mol.
Is ClF3 Polar or Nonpolar ? دیدئو dideo
Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\)

How to draw ClF3 Lewis Structure? 4
Figure 6.2.1 (a) The distribution of electron density in the HCl molecule is uneven. The electron density is greater around the chlorine nucleus. The small, black dots in the center of the green spheres indicate the location of the hydrogen and chlorine nuclei in the molecule. (b) Symbols δ+ δ + and δ− δ − indicate the polarity of the H.
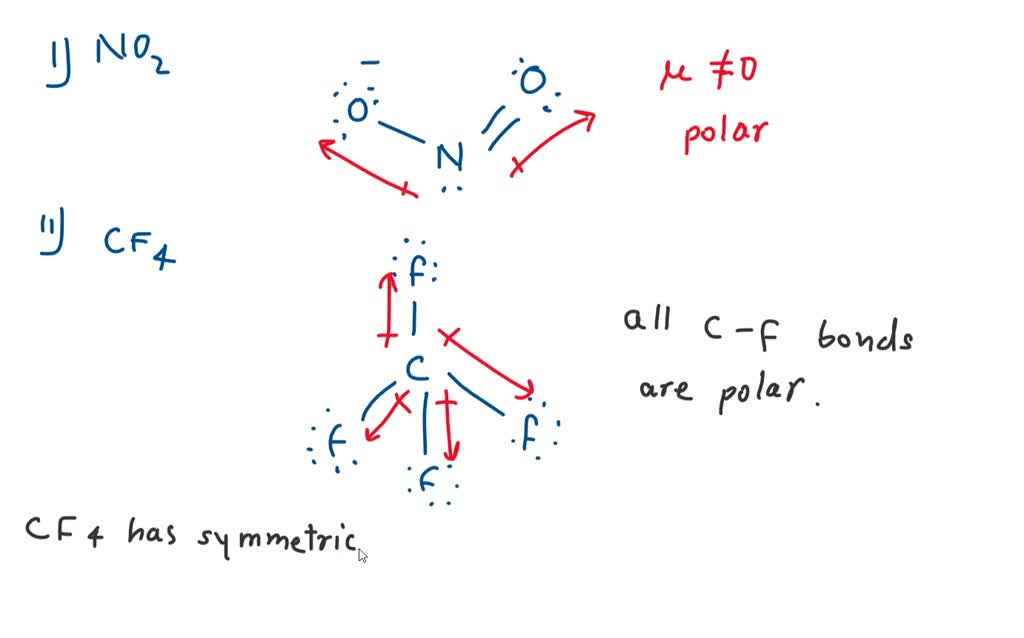
SOLVED Which molecule has polar bonds but is nonpolar? A. ClF3 B. H20
Expert-verified. Step 1. ANSWER. ClF A 3 molecule is Polar. View the full answer Step 2. Unlock. Step 3. Unlock. Answer.
Clf3 Polar Or Nonpolar Asking List
Learn to determine if ClF3 (Chlorine trifluoride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lew.

BrF3 Polar or Nonpolar? (Bromine Trifluoride) Molecules, Chemical
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.

Lewis Structures, VSEPR and Polarity Notes YouTube
How to draw lewis structure for ClF3? ClF3 lewis structure comprises three fluorine (F) atoms and one chlorine (Cl) atom. The chlorine (Cl) atom is kept at the central position and the fluorine (F) atoms are in the surrounding position in the lewis diagram. The lewis dot structure of ClF3 contains total of 11 lone pairs and 3 bond pairs.

Is ClF3 Polar or Nonpolar (Chlorine Trifluoride) YouTube
ClF is a POLAR molecule because any two bonding atoms whose electronegativity difference is between 0.4 to 2.0 forms a polar bond. Here in ClF molecule, the electronegativity difference of Chlorine atom (Cl = 3.16) and Fluorine atom (F = 3.98) is 0.82 (i.e 3.98 - 3.16 = 0.82).

CLF3 Lewis Structure, Molecular Geometry, and Polarity What's Insight
Answer: ClF3 is a polar molecule due to the presence of two pairs of lone pair electrons. The resulting electron-electron repulsion causes a bent structure, leading to an unequal distribution of charge. This induces a permanent dipole.

Free download hd /best overview is ch4 polar or nonpolar science
ClF3 is polar. The physical shape of the molecule is almost T-shaped and therefore given the two lone pairs of electrons also present on the molecule, the charge does not centre itself on the.

ClF3 is a polar and planar molecule Chemistry Chemical Bonding and
ClF3 is a polar compound. sicl4 polar or nonpolar Is Nh3 Polar? Frequently Asked Questions